In silico analysis of alternative splicing on drug-target gene interactions
Yanrong Ji, Rama K. Mishra & Ramana V. Davuluri. In silico analysis of alternative splicing on drug-target gene interactions. Scientific Reports (2020)
How they used Xena
They used Xena’s Transcript View to compare transcript-level expression between tumor and normal samples, as well as generating figures 4 and 6.
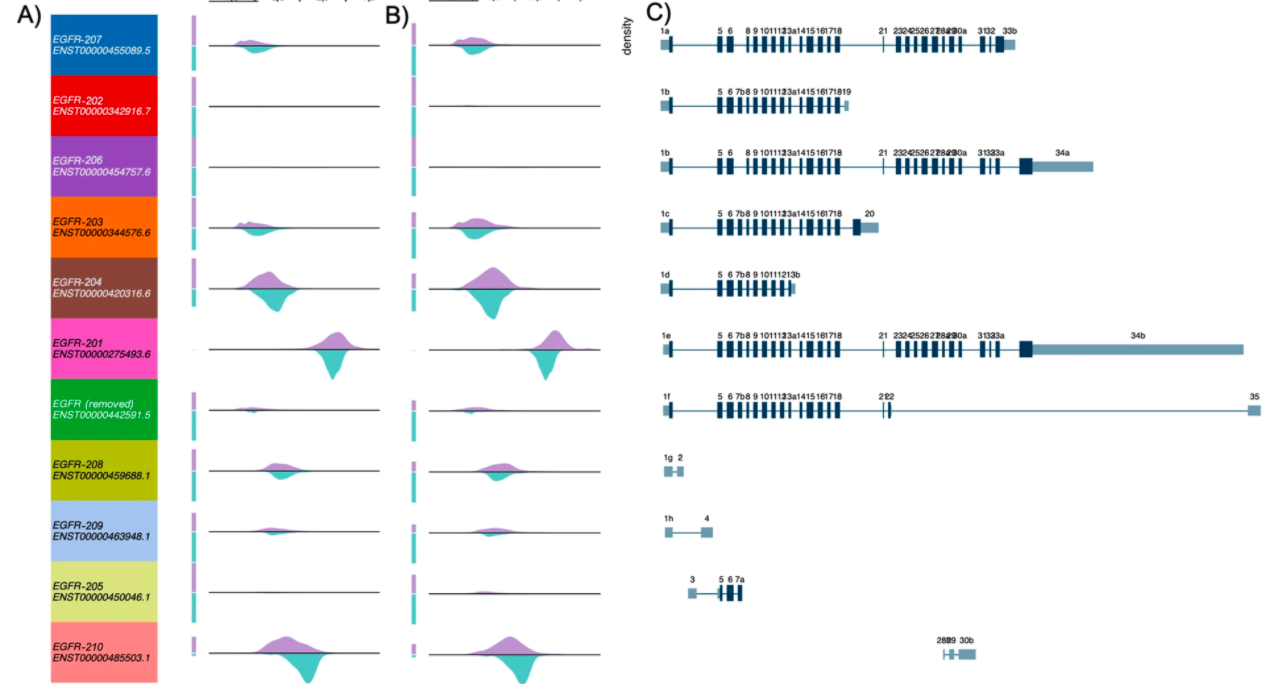
Figure 4. Examining differences in EGFR transcript expression between TCGA Lung Squamous Cell Carcinoma (LUSC) samples to GTEx normal lung samples using Xena’s Transcript View.
Paper
In silico analysis of alternative splicing on drug-target gene interactions
Identifying and evaluating the right target are the most important factors in early drug discovery phase. Most studies focus on one protein ignoring the multiple splice-variant or protein-isoforms, which might contribute to unexpected therapeutic activity or adverse side effects. Here, we present computational analysis of cancer drug-target interactions affected by alternative splicing. By integrating information from publicly available databases, we curated 883 FDA approved or investigational stage small molecule cancer drugs that target 1,434 different genes, with an average of 5.22 protein isoforms per gene. Of these, 618 genes have ≥5 annotated protein-isoforms. By analyzing the interactions with binding pocket information, we found that 76% of drugs either miss a potential target isoform or target other isoforms with varied expression in multiple normal tissues. We present sequence and structure level alignments at isoform-level and make this information publicly available for all the curated drugs. Structure-level analysis showed ligand binding pocket architectures differences in size, shape and electrostatic parameters between isoforms. Our results emphasize how potentially important isoform-level interactions could be missed by solely focusing on the canonical isoform, and suggest that on- and off-target effects at isoform-level should be investigated to enhance the productivity of drug-discovery research.